Click here to erase all data entry on this page
7-3: Analysis of a Ferrous Chloride Sample
Titrations provide a method of quantitatively measuring the concentration of an unknown solution. This is done by delivering a titrant of known concentration into an analyte of known volume. (The concentration of an unknown titrant can also be determined by titration with an analyte of known concentration and volume.) In oxidation-reduction (redox) titrations, the voltage resulting from the mixture of an oxidant and reductant can be measured as the titration proceeds. The equivalence point of the titration, or the point where the analyte has been completely consumed by the titrant, is identified by the point where the voltage changes rapidly over a small volume of titrant delivered. In this assignment, you will determine the mass % of an unknown sample of ferrous chloride (FeCl2) by titrating it with a KMnO4 solution of known concentration.
- To start this activity, click this link for Analysis of a Ferrous Chloride Sample. The lab will load in a new tab. Click back to this tab to read further instructions and complete the questions below.
- Click the Beakers drawer and double click on a beaker to move it to the spotlight next to the balance. Move the bottle of FeCl2 to the Balance area also. Click on the Balance area to zoom in, open the bottle of unknown FeCl2 by clicking on the lid (Remove Lid). Drag the beaker to the balance to place it on the balance pan and tare the balance. Click the image of the largest sample size on the front of the bottle and drag the scoop to the beaker on the balance until it snaps in place and then let go. Repeat this again so you have approximately 2 g of unknown in the beaker. Record the mass of the sample in the data table below. Close the balance view.
- Place the beaker on the stir plate. Drag the 50 mL graduated cylinder under the tap in the sink and fill it with distilled water. It will automatically snap back into place when it is full. Drag the full 50 mL graduated cylinder to the beaker on the stir plate and then pour the water into the beaker. Now place the voltmeter probe in the beaker and make sure the voltmeter is on in the Live Data display.
- The buret will be filled with 0.0815 M KMnO4. Click the Save Data button in Live Data so the titration data will be saved. The horizontal position of the orange handle is off for the stopcock. Open the stopcock by pulling down on the orange handle. The vertical position delivers solution the fastest with three intermediate rates in between. Turn the stopcock to one of the fastest positions. Observe the titration curve. When the blue line begins to turn up, double-click the stopcock to turn it off. Move the stopcock down one position to add volume drop by drop.
There are two methods for determining the volume at the equivalence point: (1) Stop the titration when a color change occurs. Click the Stop Saving button. Open the Lab Book tab and you'll see a table with all the data from the titration. Scroll down to the last data entry and record the volume at the equivalence point in the data table. OR (2) Add drops slowly through the equivalence point until the voltages reaches a maximum and levels off. Click the Stop Saving button. Open the Lab Book to see the data table. Click the Copy Data button to copy and paste the data into a spreadsheet program. Plot the first derivative of voltage vs. volume. The peak will indicate the volume at the equivalence point since this is where the voltage is changing the most rapidly as the volume changes.
- Repeat the titration at least two additional times, recording your data in the data table. Do not forget to refill the buret with KMnO4, place the voltmeter probe in the beaker, and add water each time. You can click the link in step 1 above to reset the lab.
The molecular weight of FeCl2 is 126.745 g/mol.
Data Table
| Trial | mass FeCl2 (g) | volume KMnO4 (ml) | molarity KMnO4 (mol/L) |
| 1 | |||
| 2 | |||
| 3 |
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 2: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 3: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 4: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 5: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 6: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 7: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 8: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 9: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
6. Write a balanced net ionic equation for the reaction in acidic solution of FeCl2 and KMnO4 (Fe2+ is oxidized to Fe3+ and MnO4- is reduced to Mn2+).
Box 1: Enter your answer as text. This question is not automatically graded.
- The moles of MnO4- can be calculated by multiplying the volume of MnO4- required to reach the endpoint multiplied by the molarity of the MnO4- solution.
What are the moles of MnO4- used in the titration?
| moles MnO4- (mol) |
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 2: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 3: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
- The moles of FeCl2 can be calculated by using the mole ratio from the balanced equation.
How many moles of FeCl2 were in the unknown?
| moles FeCl2 (mol) |
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 2: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 3: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Do It Yourself - Graphing and Plotting Data
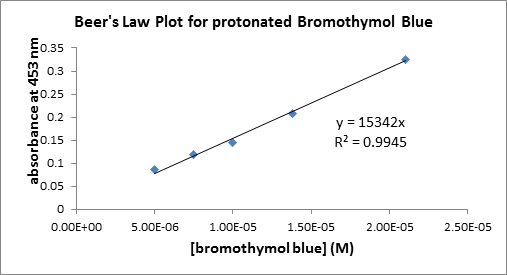
When bromothymol blue, an acid/base indicator, is dissolved in dilute hydrochloric acid it turns yellow in color. The data in the table below was collected to determine the relationship between absorbance of this protonated yellow species at `453" nm"` and its concentration. In a buffered green solution of bromothymol blue, the amount of the protonated version can be determined by measuring the absorbance at `453 " nm"`. For the green solution, the absorbance at `453" nm"` was `0.177" a.u."` (Recall that `A= epsilon bc`)
| [bromothymol blue] M | A at `453text( nm)` |
|---|---|
| 5.00E-06 | `0.086` |
| 7.50E-06 | `0.119` |
| 1.00E-05 | `0.145` |
| 2.10E-05 | `0.325` |
| 1.38E-05 | `0.208` |
The concentration of the protonated yellow species is: `text( M)`
Hint 1
Use excel or similar graphing program to plot the data such that absorbance is on the y-axis and concentration is on the x-axis, determine the linear least square fit to the data ( note that the intercept should equal zero):
Box 1: Enter your answer as a number (like 5, -3, 2.2172) or as a calculation (like 5/3, 2^3, 5+4)
Enter DNE for Does Not Exist, oo for Infinity
- The mass % of FeCl2 in the unknown sample can be calculated by dividing the mass of FeCl2 in the sample by the total mass of the unknown sample.
What is the % FeCl2 in your unknown sample?
| % FeCl2 (%) |
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 2: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 3: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
- What is the average % FeCl2 in the unknown sample using your three answers?
%
Open the Unknowns tab in the tray, expand the Assigned Unknowns menu and enter your calculated %. Click Submit. Report the actual result that the program tells you you had here. That is also recorded in your LabBook.
Actual result: % FeCl2
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 2: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Box 3: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity