Click here to erase all data entry on this page
Chemical Kinetics- Determining the Rate Constant of a Crystal Violet Reaction
Background
Chemical reactions have rates – speeds – at which they proceed. By monitoring a reaction and knowing the concentrations of the reactants, the rate of reaction can be calculated. The general rate law for a reaction that has two reactants is as follows,
rate =k[X]m[Y]n
For this experiment the reaction of interest is that of crystal violet dye with OH- (hydroxide).
The crystal violet cation (CV+) is purple in solution. The addition of OH- (via NaOH) to the crystal violet molecule causes the solution to become colorless.
To measure how quickly this reaction occurs, you can monitor the disappearance of the purple color using a spectrometer. A spectrometer is an instrument used to measure the intensity of light that enters it. When shining light through a purple solution of crystal violet, the absorbance will decrease over time, as the crystal violet reacts with the OH- and the solution becomes colorless.
We can relate the absorbance to the concentration following Beer's law:
A =εlc
where A is absorbance, ε is the absorption coefficient, l is the path length (1 cm), c is the concentration (in M). ℇ for crystal violet, at the wavelength you'll be using, is 50,000 1/(M·cm).
The rate of the reaction of CV+ with OH- can be measured by monitoring the time it takes for the solution to become colorless.
CV+ + OH- → CVOH
In this specific activity, we will tell you that the order with respect to OH- is 1. The order with respect to CV+ is also 1. We have other activities where you can calculate those orders of reaction yourself.
The rate law for this reaction is:
rate =k[CV+]1[OH-]1
In this activity OH- will be added to the reaction in excess, so its concentration will change little (relatively) while the CV+ is consumed. This means we can treat [OH-] as a constant and factor it into k to get a k’ (the pseudo rate constant) like this:
k' =k[OH-]
so the rate law simplifies to:
rate =k'[CV+]
The integrated rate law for crystal violet in this reaction is
ln[CV+] = -k't + ln[CV+]0
Where t is time and [CV+]0 is the initial concentration of crystal violet.
In this video we'll do an overview of how to perform this lab.
Procedure
- Click this link to load the lab already set up for you: Rate Constant of a Crystal Violet Reaction. The lab will load in a new tab. Click back to this tab to read further instructions and complete the questions below.
- In Live Data, you can see the absorption spectrum for water, the “blank” for this experiment, and it will be subtracted from other collected spectra.
- Click on the Stockroom tab in the tray and then on the little trash can next to Liquids to remove the water sample.
- Expand the Liquids menu and then the Kinetics menu. Click on the CV+ 0.12 sample option. Leave it on the stockroom shelf. This sample has 0.12 M NaOH in it, and at the moment you place it on the table a small volume of 5 x 10-6 M crystal violet will be added and the reaction will begin.
- Click on the Live Data tab. On the left of the graph, switch the axis from Intensity to Absorbance. At the bottom, toggle from Full to Visible. You will be recording the absorbance of the solution at a wavelength around 594 nm. These next few steps will need to be completed fairly quickly so that you can begin recording as close to the beginning of the reaction as possible. You will do at least one trial slowly just to get a feel for the process, then you will need to do it quickly.
|
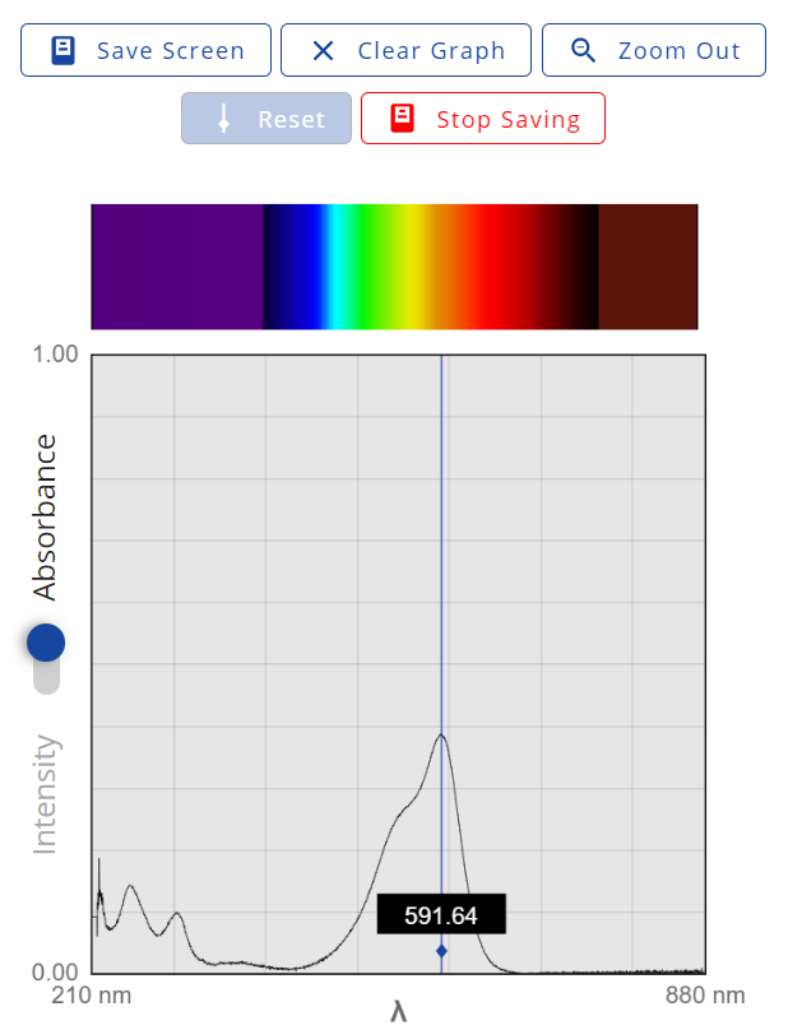 |
- As soon as you have completed these trial steps, you can click Stop Saving and go back to the Stockroom and click the Liquids trash can icon to discard that CV+ solution.
- When you are ready to repeat the experiment, select the CV+ 0.12 sample from the Liquids menu again. Drag it between the source and the spectrometer and complete the steps outlined in 6 quickly to save the absorbance data for the reaction. Let the reaction run for about 2 minutes, at which point you can click Stop Saving.
- Click on the Lab Book tab, where your data is stored. You will see a data table from the run you just completed. Click the Copy Data button to record the data to the clipboard. Paste it into a spreadsheet program, and use Beer's Law to calculate the concentration of CV+ ([CV+]) from the given values for l and ℇ and the Absorbance values you just measured. Then add another column and in it take the natural logarithm of each [CV+] value.
- Finally, make a graph with the time data on the x axis and the ln[CV+] data you just calculated on the y axis. You have now plotted the data in the same format as this equation: ln[CV+] = -k't + ln[CV+]0 . You can see that the negative slope of this graph is equal to the value k’. Use the spreadsheet program to find the slope of your data and report it as the value of k’.
k'=
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Now solve for k.
Use the equation: k' =k[OH-], which relates the pseudo rate constant k', to the actual rate constant k, along with the concentration of OH- used in this reaction (0.12 M).
k=
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Repeat the experiment twice more with the same sample (CV+ 0.12) and calculate k’ and k for each trial, then take the average of your three rate constants and report it here.
Average k:
Save your spreadsheet with all of your data analysis and submit that to your instructor so they can see your calculations and graphs.
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity