Click here to erase all data entry on this page
Recrystallizing a Compound and Determining its Melting Point
Often in an organic chemistry lab, chemists are most interested in obtaining a compound in its purest form. It is easier to identify a pure compound than an impure one. To improve the purity of a solid compound, organic chemists will often recrystallize it. Crystals form when identical molecules or atoms come together to build a repeating geometrical pattern, called a lattice. Because a crystal lattice is made up almost entirely of identical molecules, crystals are very pure forms of the compounds that comprise them.
Recrystallization can be done in a variety of simple ways. Just a few possible techniques will be covered here. The first recrystallization technique involves saturating a hot solvent with the compound of interest. A minimal amount of compatible solvent is added to an amount of solid in a beaker. The mixture is gently heated and stirred until the solid has completely dissolved. At this time, the solution is removed from heat so that it may cool. As the solution cools, the solubility of the compound in the solvent decreases, and crystals of the compound will begin to precipitate. These crystals can be filtered from the solvent for analysis.
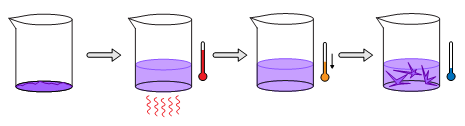
Single Solvent, Hot–Cold Method
Another crystallization technique allows the solvent in a solution of the compound to slowly evaporate. Enough solvent is eventually evaporated to precipitate crystals of the compound. If the solvent is highly volatile, a cap with small holes may be placed above the solution to prevent rapid evaporation.
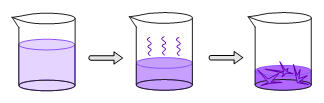
Slow Evaporation Method
The final crystallization technique uses two miscible solvents to precipitate the compound. The compound is soluble in one of the solvents but not the other. A solution of the compound is made with the solvent in which it is soluble. The other solvent, called the counter solvent, is then slowly added to the solution. As the two solvents mix, the solubility of the compound in the mixture decreases. When enough counter solvent is added, the compound will precipitate.
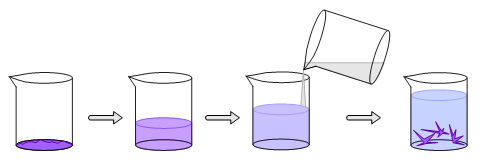
Counter Solvent Method
After a compound is recrystallized, its melting point range is often used to determine its purity. Impurities within the compound or mixtures will lead to melting points outside the expected range for the pure compound.
The simplest way to determine a melting point range is to use a melting point apparatus. A few milligrams of the sample to be analyzed are added to a capillary tube (a very small, thin, glass tube with an opening at one end). The tube is then placed in the analysis well of the melting point apparatus. The rate of temperature increase can be controlled and set by the user. Often, a small magnifying glass provides a view of the bottom of the capillary tube while it sits in the analysis well. The melting point range for the compound begins at the temperature when small beads of liquid begin to form on the wall of the capillary. The end of the melting point range is the temperature at which the entire sample has liquified. The determined melting point range can then be compared to reported values for the compound in its purest form.
Beyond Labz Organic Synthesis uses simplified simulations for recrystallization and melting point determination. In this assignment, you will be guided through the steps of a recrystallization and a melting point determination for the products of a benzene nitration reaction. This assignment will also serve as a tutorial to teach you how to utilize the various parts of the organic simulation that will be used in later assignments.
If at any point you need to exit the lab and come back at another time, click the Save Lab button below. That will save your progress. Then when you re-enter the lab, open the Presets menu and click on My Saved Labs. You can click the latest link there to start at the point where you left off.
- To start this activity, click this link for Recrystallizing a Compound and Determining its Melting Point. The lab will load in a new tab. Click back to this tab to read further instructions and complete the questions below. You can follow along with the instructions below in the Procedures tab in the lab. You will see a lab bench containing reagents on the back of the bench, aqueous reagents on the right, an equipment rack containing necessary laboratory equipment, a red disposal bucket for cleaning up the lab, and the organic stockroom in the back. Other pieces of laboratory equipment will be used in other assignments.
- You will find a round bottom flask on the cork ring that contains three functionalized aromatic products. The product of interest for this assignment is 1-methyl-4-nitro-benzene. Use the internet to find a melting point for this compound and record your source(s).
Melting point: °C
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Source(s):
Box 1: Enter your answer as text. This question is not automatically graded.
In this video we'll show you how to use the distillation apparatus.
3. Move the flask onto the stir plate and perform a distillation (see activity Performing a Distillation). One of the three products will distill as a liquid, and the other two will remain in the distillation flask as solids.
What two products comprise the solid?
Box 1: Enter your answer as a list of text separated by commas. Example: dog, cat, rabbit.
In this video we'll show you how to perform a recrystallization.
4. Remove the distillation apparatus by dragging it to the equipment rack. The distillation flask with the solids should remain on the stir plate. Now drag the distillation flask to the Crystallization dish, located on the right of the lab bench, just below the equipment rack. This step will automatically perform a full recrystallization in abbreviated time. A yellow crystalline solid should appear in the dish. If the crystals are pure 1-methyl-4-nitrobenzene, the melting point you determine next should match that which you found reported in Question 2.
5. Click on the melting point apparatus (the small black instrument at the bottom right of the instrument bench) and drag a capillary tube to the crystallization dish. Now hover over the small LED display on the apparatus, and the melting point should display in larger text. You can also see the melting point recorded in the Live Data display. If the display just has asterisks (stars) that means that the mixture is a liquid at room temperature (the melting point is less than 25°C).
What is the determined melting point for the solid?
°C
Box 1: Enter your answer as letters. Examples: A B C, linear, a cat
Does this value match your reported value? Why or why not?
Box 1: Enter your answer as text. This question is not automatically graded.
6. Now open this preset: Recrystallization Part 2. The lab should reset, and a new round bottom flask should be sitting on the cork ring. This flask contains two of the products you saw in the first part of the activity and one new compound.
7. Move the flask onto the stir plate and perform a distillation (see activity 1-3: Performing a Distillation). Two of the three products will distill as liquids, and the other will remain in the distillation flask as a solid.
8. Remove the distillation apparatus by dragging it to the equipment rack. The distillation flask with the solid should remain on the stir plate. Now drag the distillation flask to the Crystallization dish, located on the right of the lab bench, just below the equipment rack. This step will automatically perform a full recrystallization in abbreviated time. A yellow crystalline solid should appear in the dish. If the crystals are pure 1-methyl-4-nitrobenzene, the melting point you determine next should match that which you found, reported in Question 2.
9. Click on the melting point apparatus (the small black instrument at the bottom right of the instrument bench) and drag a capillary tube to the crystallization dish. Now hover over the small LED display on the apparatus, and the melting point should display in larger text.
What is the determined melting point for the solid?
°C
Box 1: Enter your answer as an integer or decimal number. Examples: 3, -4, 5.5172
Enter DNE for Does Not Exist, oo for Infinity
Does this value match your reported value? Why or why not?
Box 1: Enter your answer as text. This question is not automatically graded.